Scientists at the Sandia National Laboratory have developed a “memzyme,” a membrane nearly saturated with carbonic anhydrase, an enzyme cells use to rid themselves of carbon dioxide quickly and efficiently. The patented work has grabbed the attention of energy companies that would like to significantly and inexpensively reduce carbon dioxide emissions, one of the most widespread greenhouse gases, and explore other possible uses of the invention.
The memzyme meets the Department of Energy’s standards by capturing 90% of power plant’s carbon dioxide production at a relatively low cost of $40 per ton. “To date, stripping carbon dioxide from smoke has been prohibitively expensive using the thick, solid, polymer membranes currently available,” says Jeff Brinker, a Sandia fellow. “Our inexpensive method follows nature’s lead in its use of a water-based membrane only 18 nanometers thick that incorporates natural enzymes to capture 90% of carbon dioxide released.
“This is almost 70% better than current commercial methods, and it’s done at a fraction of the cost,” Brinker continues.
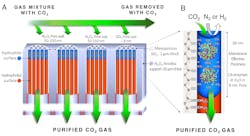
The carbon-dioxide grabbing co membrane is fabricated by first forming 8-nanometer diameter mesopores. Using atomic layer deposition and oxygen plasma processing, the silica mesopores are engineered to be hydrophobic except for an 18-nm-deep region at the pore surface which is hydrophilic. Through capillary condensation, carbonic anhydrase enzymes and water spontaneously fill the hydrophilic mesopores to form an array of stabilized enzymes with an effective concentration greater than 10 times of that achievable in solution. These catalyze the capture and dissolution of carbon dioxide at the upstream surface and the regeneration of carbon dioxide at the downstream surface. The high enzyme concentration and short diffusion path maximizes capture efficiency and flux. (Image courtesy of Sandia National Laboratories)
Coal power plants are one of the United States’ largest energy producers, but they have been criticized by some for sending more carbon dioxide into the atmosphere than any other form of electrical power generation. Still, coal burning in China, India, and other countries means that U.S. abstinence alone is not likely to solve the world’s climate problems.
The device’s formation begins with a drying process called evaporation-induced self-assembly, first developed at Sandia by Brinker 20 years ago. The procedure creates a closely-packed array of silica nanopores that will hold carbonic anhydrase enzyme and keep it stable. This is done in several steps. First, the array, which may be 100 nm long, undergoes atomic layer deposition to make the nanopore’s surface water-averse or hydrophobic. This is followed by an oxygen plasma treatment that covers the water-averse surface to make the nanopores water-loving or hydrophilic, but only to a depth of 18 nanometers. A solution of the enzyme and water spontaneously fill up and are stabilized within the water-loving portion of the nanopores. This creates membranes of water 18 nanometers thick, with a carbonic anhydrase concentration 10 times greater than aqueous solutions made to date.
The solution, at home in its water-loving sleeve, is stable. But the enzyme can rapidly and selectively dissolve carbon dioxide, so the catalytic membrane captures most of the carbon dioxide molecules that brush up against it in a rising cloud of coal smoke. Captured molecules pass rapidly through the membranes, driven solely by the naturally occurring pressure gradient caused by the large number of carbon dioxide molecules on one side of the membrane and their comparative absence on the other. The chemical process turns the gas briefly into carbonic acid and then bicarbonate before exiting immediately downstream as carbon dioxide gas.
The gas can be harvested with 99% purity, so pure it could be used by oil companies for resource extraction. Other molecules pass the membrane’s surface undisturbed. The enzyme is reusable, and because the water serves as a medium rather than an actor, does not need to be replaced.
The nanopores dry out over time due to evaporation. This will be checked by water vapor rising from lower water baths already installed in power plants to reduce sulfur emissions. And enzymes damaged from use over time can easily be replaced.
“The high concentration of carbonic anhydrase, along with the thinness of the water channel, result in high carbon dioxide flux through the membrane,” explains Brinker. “The greater the carbonic anhydrase concentration, the greater the flux. The thinner the membrane, the greater the flux.”
The membrane’s arrangement in a generating station’s flue would be like that of a catalytic converter in a car, suggests Brinker. The membranes would sit on the inner surface of a tube arranged like a honeycomb. Flue gas would flow through the membrane-embedded tube, with a carbon dioxide-free gas stream on the outside of the tubes. Varying the tube length and diameter would adjust the carbon dioxide extraction process.
“Energy companies and oil and gas utilities have expressed interest in adjusting gas filters for specific conditions,” says Susan Rempe, a Sandia researcher. “The enzyme can catalyze the dissolution of a million carbon dioxide molecules per second, vastly improving the process’ speed. With improvements by Industry, the memzyme could make electricity production cheap and green.”
The separation process could increase the amount of fuel obtained by enhanced oil recovery using carbon dioxide injected into existing reservoirs.
A slightly different enzyme, used in the same process, converts methane—an even more potent greenhouse gas than carbon dioxide—into the more soluble methanol for removal.
The procedure also could sequester carbon dioxide on a spacecraft because the membranes operate at ambient temperatures and are driven solely by chemical gradients.