FDA Approves Implant for Adults with Above-the-Knee Amputations
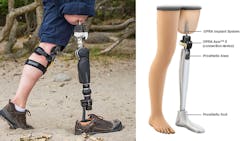
The U.S. Food and Drug Administration has approved the osseoanchored (bone anchored) prostheses for rehabilitating amputees (OPRA) implant developed by biomedical engineers at Integrum AB in Sweden. It is the first implant approved by the FA in the U.S. for adults who have above-the-knee amputations, and who have or could have problems with conventional socket prosthetics.
Conventional leg prosthetics use a fitted, cup-like shell called a socket that fits over the remaining portion of the patient’s leg to secure the lower-leg replacement. Some patients may not have enough of a residual limb to hold a socket prosthetic, or may have scarring, pain, recurrent skin infections or ongoing changes in the residual limb’s shape that prevents them from using one.
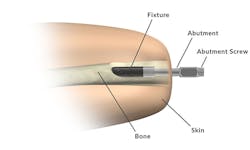
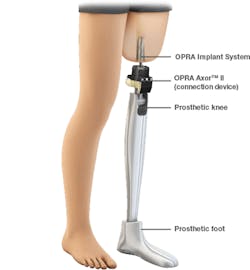
The new OPRA is implanted in two surgical procedures. In the first, a cylindrical titanium fixture is implanted into the central canal of the remaining femur. About six months later, after bone tissue has grown into the implant to anchor it and the skin has healed, the next procedure attaches the OPRA implant to the bone-anchored fixture implanted in the previous surgery. The implant extends through the skin at the bottom of the patient's residual limb and connects to the prosthesis.
After the second surgery, the patient works with a trained physical therapist to gradually place weight on the implant over about six months of training and rehabilitation. After that, they are fitted with a customized prosthesis by a trained prosthetist.