Supplier Collaboration is Key to Developing Medical Devices
Medical device companies are under extreme pressure to deliver new and improved products to the market ahead of the competition. While the overall process for a medical device takes years, most of that time is devoted to lead times for tooling and components, market feedback, life testing and seeking regulatory approvals. Engineers must often wait to update a design until these tasks are completed, leaving a tight window to complete their next deliverables before the deadline.
Immediately after management greenlights a project, engineers are expected to rapidly build proof-of-concept (POC) samples for internal review and market feedback, with speed prioritized over perfection. Weeks or months later, finance approves funding and marketing confirms the target specifications, at which point pressure is once again on the engineers to quickly turn out more durable and sophisticated prototypes for further testing. This “hurry-up-and-wait” cycle repeats itself through verification testing and regulatory approval.
Unfortunately, this time pressure often compromises the final product. To meet early stage deadlines, it is a common and accepted practice to design POC samples around readily available standard components with the intention of fine-tuning the design by using customized parts in the next round of prototyping. However, similar time pressure during subsequent iterations can make lead times for custom components prohibitive. As a result, concessions made on even the most rudimentary prototypes frequently survive to production, lowering product performance and increasing total costs.
Staying on track while delivering a high-quality design requires choosing supply partners that can help manage the evolution of a product as it goes from concept to launch. The suppliers must be able to quickly turn out standard prototypes for developing the product concept and also quickly customize parts for future iterations. The suppliers must be able to do all this at the right price and quality levels.
It is best to use one supplier throughout the process because it will learn more from testing the concept if its own products are used, and it can execute each stage while keeping the big picture in mind. If a supplier not viable for production must be chosen for the concept phase, it is critical to also work with a strategic supplier at the same time. An effective strategic supply partner can help engineering teams prepare to use the custom component by consulting on the concept and beginning work on customization at the same time.
The importance of supplier selection during early development stages is compounded by the fact that most medical devices cannot qualify several sources for critical components. As deadlines approach and issues arise, designers are forced to eliminate as many moving parts as possible.
Trying to get competing suppliers to meet the same specification and timeline is an added challenge that can rarely be supported. As a result, medical products are typically submitted for regulatory approval with only one supplier per component. Qualifying secondary suppliers after production has started rarely happens because it requires resubmission to regulators, a prospect generally rejected by management because it is too expensive, eats up too many resources and is too risky. And of course, qualifying two suppliers means double the development cost, and purchasing fewer products from two suppliers reduces volume pricing.
Given the difficulty in qualifying two suppliers and in switching suppliers at any point during the process, it is critical to choose strategic supply partners at the concept phase based on whether they can:
- Manufacture standard products designed for the POC prototype and shipment within a matter of days.
- Demonstrate the industry experience to anticipate and resolve common traps while contributing design suggestions for improvement.
- Supply design and manufacturing engineers and customer collaboration at all stages.
- Support building medical devices with its prototyping and product development capabilities.
- Provide facilities with good validation and change-control processes that regularly pass quality audits of leading medical device companies.
- Outline a clear plan to meet the target production price.
- Use a wide array of technology options that does not restrict product design.
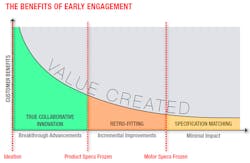
To take full advantage of such a supplier’s capabilities, the relationship must begin at the concept or even ideation stage. The right supply partner will be able to provide valuable insight to choose the best path from the outset. Just a few hours spent upfront with the supplier’s experts can significantly reduce future design hours, cut overall development time by months and ultimately deliver a better, more valuable product to the market.
To illustrate the benefits of concept phase collaboration with a strategic supply partner, consider the following example of an engineering team selecting a supplier for the motor in a medical device. The team needs to have a proof of concept in three weeks, complete tests for regulatory submission in six months and be in high-volume production in two years. The POC clearly dictates an off-the-shelf motor, but the design team chooses a concept stage supplier it knows can also deliver the necessary custom version while meeting the price and quality level for production.
Within days or even hours of initial contact, the motor supplier calls to discuss the design’s long-term goals. It recommends a standard motor that can be delivered within a week and is already tailored to the application based on the supplier’s industry experience. Modifying standard products to meet common customization requests leads to POC builds that are closer to the final design criteria. This can avoid an extra iteration before finalizing the design freeze, potentially saving weeks or months of unnecessary engineering effort and thousands of dollars. Any remaining customizations needed—such as output shaft configurations, dialed-in windings and gear ratios—are planned during this initial discussion so the design team can account for differences during POC testing.
Once POC testing is complete, a strategic motor supplier can then provide custom prototypes in as little as four weeks when there is a development strategy that preemptively initiates design work and long lead time component or tooling. This leaves the device designer with ample time to complete design work, iterate on the motor design or resolve other issues before the six-month regulatory filing deadline. After testing is completed on each iteration of prototypes, the motor supplier sends its engineers to the customer’s site for detailed design reviews of the motor and system.
When it comes time to move to production, it is assured that the motor supplier can execute the design. If engineering needs to switch suppliers at this time, it is unlikely a new supplier would meet the application’s requirements without a design change that would derail regulatory approval.
In the end, successfully using a custom motor maximizes power and efficiency while eliminating extra components, therefore reducing costs, size and complexity. The total development time is also shortened, thanks to the seamless transition between motor iterations and eliminating surprises that could have rippled through the design.
Brandon Steinberg is senior manager of business and strategy for Surgical Moto Solutions for Portescap.