Fuel cells have not been particularly known for practicality and affordability, but that may have just changed, thanks to developments in a lab at The Georgia Institute of Technology. A team of researchers there recently developed a new fuel cell that runs on cheap fuel at temperatures comparable to automobile engines, and which slashes materials costs. The key to its fuel cell was the discovery of new catalyst.
The catalyst does away with priced hydrogen fuel by making its own out of cheap, readily available methane. And improvements throughout the cell dramatically cooled the operating temperatures customary in methane fuel cells. Methane fuel cells usually require temperatures of 750 to 1,000°C. The Georgia Tech fuel cell needs only about 500°C—a notch cooler than automobile combustion engines, which run at around 600°C.
That lower temperature could trigger cascading cost savings in the ancillary technologies needed to operate a fuel cell, potentially pushing the new cell to commercial viability. The researchers express confidence that engineers can design electric power units around this fuel cell, something that has eluded previous methane fuel cells.
“Above 750°C, no metal would withstand the temperature without oxidizing, so you’d have a lot of trouble getting materials, and they would be extremely expensive and fragile, and contaminate the cell,” says said Meilin Liu, the Georgia Tech professor who leads the team. “Lowering the temperature to 500°C is a sensation in our world. Very few people have even tried it. When you get that low, it makes the job of the engineer designing the stack and connected technologies much easier.”
The new cell also eliminates the need for a major add-on called a steam reformer, which is normally required to convert methane and water into hydrogen fuel.
The research was based on a type of fuel cell with high potential for commercial viability, the solid oxide fuel cell (SOFC). SOFCs are known for their versatility in using different fuels. If the new fuel cell goes to market, it still might not power automobiles for a while. Instead it could first land in basements as part of a more decentralized, cleaner, cheaper electrical power grid. The fuel cell would be about the size of a shoebox, not counting the other equipment needed to make it run.
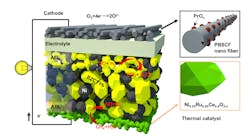
Cascading innovations, including a brand new one, let researchers reimagine the fuel cell, making it run on methane at lower temperatures. The ruthenium-nickel based catalyst, here in green, was the latest materials innovation in the new fuel cell. (Credit: Georgia Tech/Liu lab)
“The hope is you could install this device like a tankless water heater,” Liu says. “It would run off of natural gas to power your house…That would save society and industry the enormous cost of new power plants and large electrical grid expansions.
“It would also make homes and businesses more power independent,” he adds. “That kind of system would be called distributed generation, and our sponsors want to develop that.”
Hydrogen is the best fuel for powering fuel cells, but its cost is exorbitant. Researchers figured out how to convert methane to hydrogen in the fuel cell itself via the new catalyst, which is made with cerium, nickel, and ruthenium and has the chemical formula Ce0.9Ni0.05Ru0.05O2 (abbreviated CNR).
When methane, water molecules, and heat contact the catalyst, nickel chemically cleaves the methane molecule. Ruthenium does the same with water. The resulting parts come back together as hydrogen (H2) and carbon monoxide (CO), which researchers put to good use. Although CO causes performance problems in most fuel cells, in this one they can use it as a fuel.
H2 and CO move on to other catalyst layers that make up the anode, the part of the fuel cell that pulls off electrons, making the carbon monoxide and hydrogen positively charged ions. The electrons travel via a wire creating the electricity flow toward the cathode.
There, oxygen sucks up the electrons, closing the electrical circuit and becoming O2 ions. Ionized hydrogen and oxygen meet and exit the fuel cell as water condensation; the carbon monoxide and oxygen ions meet to become pure carbon dioxide, which could be captured.
For the energy produced, this fuel cell creates far less carbon dioxide than combustion engines.
In some fuel cells, the water in the initial reactions must be introduced from the outside. In this fuel cell, it’s replenished in the last reaction phase, which forms water that cycles back to react with the methane.
The new catalyst, CNR, manufactured by research collaborators at the University of Kansas, is the outer layer of the anode side of the cell and doubles as a protectant against decay, extending the life of the cell.
On the cathode end, oxygen’s reaction and movement through fuel cells are usually slow, but Liu’s lab has sped it up to raise the electricity output by using nanofiber cathodes, which Liu’s lab developed in a prior study. (A tailored double perovskite nanofiber catalyst enables ultrafast oxygen evolution.)
“The structures of these various catalysts, as well as the nanofiber cathodes, let us drop the operating temperature,” explains co-first author Yu Chen.