This article was originally published on MedicalDesign.com in April 2014.
The rules no longer apply. Materials do more than simply act as the foundation for form and function. Coatings are crossing boundaries to mix with materials rather than just coat them. This is creating an entire new world of possibilities for medical design engineers.
A New Tool for Designing the Smallest Medical Devices
In their focus on developing products for both short and long-term implantable medical devices, DSM biomedical has created the thinnest fiber ever to be used in the medical market.
DSM’s Dyneema Purity 10 dtex fiber holds this claim. It is the thinnest, high-strength, medical-grade ultra-high-molecular weight polyethylene (UHMWPE) fiber known today. The fiber is four times stronger than polyester and thinner than any other UHMWPE medical fiber on the market. The fiber’s slim property promotes the design of smaller devices, which can be used in support of minimally invasive surgical procedures. The typical results are less discomfort and faster recovery times for patients.
Carola Hansen, director, biomedical polyethylenes, says that production of all very thin medical fibers requires consistent quality and clean fiber material. These two issues are challenges for any fiber to begin with, but the smaller fibers are, the bigger the challenge grows. It required years of research and investment in proprietary technology before DSM launched its first UHMWPE fiber in 2004, Dyneema Purity fiber. Dyneema Purity 10 dtex fiber is the newest addition to the product portfolio. It has been fully validated and is commercially available across global markets.
A scanned electron microscope image comparing a braid of DSM fiber (below) to the point of a needle on a 1000 micrometer (µm) scale. (Courtesy of DSM Biomedical)
The fiber is available in white for use where a minimal visual profile is preferred. It is also available in blue to offer surgeons better contrast during production of medical devices and during surgical interventions. “It has low elongation and hardly stretches under tension, yet it is also flexible and soft,” Hansen adds. “Of course, it is biocompatible and is compliant with ISO 10993 standards. Our first clinical applications focus on cardiovascular sutures, used in minimal invasive interventions. This is a growing area and Dyneema Purity 10 dtex fiber allows us to expand further into the field.”
Tough Material Ensures Device and Patient Safety
Eastman Chemical Company is a global specialty chemical company that works with customers to deliver innovative products and solutions. In the medical area, one key innovation is Eastman Tritan copolyester. This is a very rigid, hard plastic that is typically used in device housings, connectors, blood oxygenators, or other parts that need a very durable material that won’t crack or break if dropped (or else degrade when exposed to chemicals or sterilization).
MonuMedical’s Flowarray line of next-generation stopcocks uses Eastman materials. (Courtesy of Eastman Chemical Company)
Emmett O’Brien, Ph.D., Eastman’s principal applications engineer, says that a number of things make this product unique: “In the medical and hospital environment, there is a significant need for plastics to be very chemically resistant. In addition, materials also need to have a specific set of properties to ensure the safety of the device and patient. Tritan does all of this.”
Tritan has the four basic properties needed for the medical environment: It’s clear, which allows health care professionals to help ensure the device is working properly. It’s also an extremely tough material, which is critical for its applications. Additionally, the properties of the material are not affected when exposed to any type of hospital aggressive disinfectants, cleaners, pharmaceuticals, or oncology drugs.
“Also when, gamma sterilization is used on other plastics, they turn very yellow and their mechanical properties can be affected,” says O’Brien. “With Tritan, there is such minimal color shift, you can’t tell the difference before and after sterilization.”
NuGen syringe line benefits from using Eastman Tritan copolyester. (Courtesy of Eastman Chemical Company)
In February, Eastman introduced the newest grade, Tritan copolyester MXF121, which offers an opaque material option. It is specifically designed to have a flammability rating necessary for certain electronics required for portable battery housings. It has the toughness of all Tritan materials but it has even better chemical resistance. Hand-held and other medical electronic device housings demand vigilance to prevent hospital-acquired infections (HAIs). Many products used to combat HAIs are chemically incompatible with thermoplastics such as PC and PC/ABS, potentially resulting in compromised performance due to cracking or crazing. MXF121 provides excellent chemical resistance against these cleansers.
Pevco uses Eastman Tritan copolyester for durable pneumatic tube carrier. (Courtesy of Eastman Chemical Company)
O’Brien says that currently, the MXF121 is Eastman’s focus, and the company is working with customers to develop more applications. “We are also working on a grade that will reduce blood clotting—anti-thrombosis. Another will reduce static so powder type medications don’t stick to the plastic itself. We are also working on even better plastic materials that will withstand autoclave sterilization.”
Winning the Fight Against Delamination in Glass Vials
Delamination is not a material or coating, but it does affect material in a very negative way. The peeling off of flakes from the inner glass surface of pharmaceutical vials as a result of interaction with the formula is a great concern. The U.S. FDA is now explicitly requiring that pharmaceutical companies manage their risks more closely. SCHOTT, an international technology group with more than 125 years of experience in the areas of specialty glasses, materials and advanced technologies, is addressing this challenge with its DC (delamination controlled) vials.
This image illustrates the evenly spread molecule structure of SCHOTT Vials DC. (Courtesy of SCHOTT AG)
“This is a whole technology,” explains Dr. Bernhard Hladik, head of product management. “Based on all the recalls which have been reported in the market due to delamination, we used our internal resources to understand the root cause of delamination. Then we developed a method to be able to measure a vial’s tendency for delamination in a very short time—a very fast, 8- to 10-hour test.”
Some may think 8 to 10 hours is very long time, but current delamination testing requires sending products out to an analytical service, taking weeks or even months, before receiving the results of the tendency of delamination.
The SCHOTT “delamination quick test” is a destructive test. It takes samples from production and puts them in an autoclave with an aggressive, corrosive atmosphere, which elaborates the critical zone of delamination.
During formation of a vial, there is evaporation of volatile components such as sodium and boron. The top walls of the vial are cool and may show condensation of sodium as white powder. This is not dangerous because it can be seen and it can be washed away. What causes delamination is that the bottom of a vial is still warm enough that the sodium and boron is actually able to get back into the glass. There is still enough heat being transferred into this area, and the material is still soft viscous enough that sodium and boron can fuse into the glass itself.
An ordinary vial with glass flakes swimming in the medication. (Courtesy of SCHOTT AG)
“Later, when it cools down, these volatile components are frozen into this specific area creating a different composition than in the rest of the vial,” says Hladik “It is sensitive to phase separation and not as stable. This means that you have an area where the delamination could start and flakes might peel off the surface.”
A microscopic image of the inner surface of an ordinary vial, showing signs of glass delamination. (Courtesy of SCHOTT AG)
SCHOTT did not change the formula for the glass. The composition was not the root cause. The group measured delamination tendency and then defined production parameter sets. Using these parameters it focused on eliminating the delamination tendency.
The SCHOTT delamination quick test takes samples from production and puts them in an autoclave with an aggressive, corrosive atmosphere, which elaborates the critical zone of delamination. (Courtesy of SCHOTT AG)
“We launched our new delamination controlled vials at the end of 2013. ISO formats are already available,” says Hladik. “We see the biggest urgency coming from the US and Europe, with the other countries following. We will continue our launch steps to meet all the required formats for all vials from 2R to 10R in the near future.”
Molding Coating Protection into the End Product
West Pharmaceutical Services is addressing the needs of medical devices in many areas. Its latest offering is FluroTec barrier film, which is applied to elastomer closures. These can be stoppers for liquid, powder or lyophilized vials, as well as components for syringe or cartridge-based technologies used with auto-injector and pen systems. It is applied to the plunger or piston that’s used to expel the drug product.
Tibor Hlobik, global director, marketing PFS Platform, explains that FluroTec is a fluropolymer film with very unique benefits. “It protects the drug in contact with the closure, but that is not the only place it is used. It can be on the top of a vial stopper. With both a top and a bottom coated with FluroTec film there is added lubricity. In some cases this can make drug filling easier and faster because products won’t stick or adhere to production line equipment.”
Lyophilization and serum stoppers coated with West FluroTec® barrier film provide remarkable advantages to the pharmaceutical manufacturer. FluroTec film provides a barrier against extractables and enhances seal integrity, important benefits to help maintain the full strength of a drug. (Courtesy of West Pharmaceuticals, Inc.)
What makes this material different is that it is part of the manufacturing and molding process, not an added coating. The FluroTec technology and manufacturing process was developed with West Pharmaceutical Services’ partner in Japan, Daikyo Seiko, Ltd. Elastomer materials must be combined with FluroTec film and then go through several stages of forming to create a finished product.
“Customers either come to us for commercially available FluroTec products or to combine FluroTec film with custom designs for their new product requirements. We are seeing more complex designs such as the latest in the Daikyo Crystal Zenith Syringe system, which consists of a Daikyo Crystal Zenith barrel with a luer lock tip sealed with FluroTec tip cap, a FluroTec fully laminated piston and polypropylene rod. We supply the complete solution,” says Hlobik.
Biologics and complex drug products with extreme pH or organic solvent systems are prime markets for this lamination technology because, as Hlobik notes, “As you start moving to these new drug products, their viscosity is also increasing and there are more constituents in every formulation. Biologics are very complex proteins with unique stability requirements. These products have very serious handling, storage, and delivery requirements to keep them working the way they should.”
From a container closer there are factors that can influence the way that protein is stable within a solution. If the biologic protein has a tendency for absorption, the drug will have lower potency. This can affect the efficacy of the drug formulation.
In absorption, the container or plunger draws material out of the formulation into itself. In that case, the drug is losing potency and may not be in the right concentration for the patient’s therapeutic need.
“A new drug may come out in a vial, move to a syringe, and then adapt to a cartridge or even move on to a more complex delivery system like a patch injector,” says Hlobik. “The pharmaceutical manufacturer needs the container material to have zero impact on the drug itself and not affect the delivery mechanism in any way. This greatly affects lifestyle management of new pharmaceuticals. FluroTec film answers all these challenges.”
This Medical Coating is a “Sandwich” that Started with a Ketchup Bottle
LiquiGlide was invented at MIT and is a patented technology platform. The coating creates an extremely slippery surface for viscous liquids. But it is not just a coating: It is a whole technology of liquid impregnated surfaces. It is a permanently wet coating.
Dave Smith, co-founder and CEO of LiquiGlide Inc., explains that the process basically traps liquid in place. “We spray on a textured solid surface and then we spray a liquid on top that gets trapped between those textures and stays trapped through capillary forces.” This is not a “one size fits all” coating solution. It is made up of different materials that are application dependent. Every application is custom.
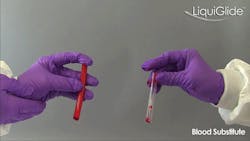
A side-by-side comparison of blood substitute evacuating from uncoated and LiquiGlide-coated flexible tubes. (Courtesy of LiquiGlide Inc.)
“We identify liquids that are compatible with the chemical and physical properties of our client’s product,” says Smith. “Once we have found a suitable liquid, we select solid materials that are promising for adhering to the client’s surface and that will form a suitable porous structure. The porous solid entraps the liquid through capillary forces. These forces are sufficient to hold the liquid in place against forces greater than 50 g. Once a prototype is in place, we create a scalable and commercially viable application process. The end result: A durable and high performing coating that meets all the requirements of our client.”
It sounds pretty straightforward. But the trick is always choosing the right solid and liquid materials for each individual application. A liquid impregnated surface that is not properly designed or matched to the product will fail because the viscous liquid will push the liquid part of the coating to the side and get stuck in the void in of the textured surface. The process works only by completely understanding the properties of the liquid or material that needs to slide across the surface, and then choosing the right solid and liquid materials for that specific product.
The work at MIT first found food-safe materials to create a coating that had the right properties with respect to ketchup. They made a ketchup bottle and that started to move the technology commercially. The medical area has a host of applications where coatings are used, and yet what they are using is insufficient. One critical example is the catheter used for brain hemorrhage.
“Right now they drill a small hole in the brain, put a thin tube into the hemorrhage and try to drain it out. But very frequently, that tube gets clogged and they have to flush it or replace it. Every time they go in and out of the brain, there is a lot of risk, and it makes the process longer. Using LiquiGlide-coated tubes, they would never need to remove or flush the drainage tube. One tube—one use from start to finish. It makes it a faster, safer process,” says Smith.
Whether blood or other bodily fluids, there is always the chance of coagulation. With LiquiGlide, coagulated fluid will still slip through the tube instead of creating a block. Smith says that they can create a product that works in any temperature for any liquid that remains a liquid and any solid that remains a solid. It’s only a matter of using materials that have the right properties with respect to the product in the container.
While not far along in the medical area yet, the technology is currently well on its way in other industries. The company has 30 clients now across many industries. Two-thirds are in consumer packaging products. Some are just in development, others are confirmed, tested and in the licensing process.
“In the medical area, we are looking for a flexible partner with the goal of establishing a relationship to use lab and facilities to conduct studies on real human blood and organs. We are currently in the process of talking with interested companies,” concludes Smith.
Ending Thoughts
The material and coating developers are keeping pace with the continually changing needs of the medical design industry. These developers, and many more like them, are keeping a finger on the pulse of industry needs to be able to deliver exactly what fills both current and future requirements.