Semiconductor nanocrystals, better known as quantum dots, are used for the bright colors they emit in for quantum-dot light-emitting diode (QLED) display screens and television. Quantum dots may be small—about the diameter of four DNA strands—but they are complex. They emit extremely pure light which can be adjusted by changing the dot’s their size, shape, composition and surface chemistry, letting them be used for display screens and TVs.
Previous studies had focused on how the dots’ electrons behaved.
But engineers have always had a problem with those dots: Attempting to get them to emit more intense light by increasing the power input always resulted in generating heat rather than more light, thus limiting the dots’ efficiency at higher intensities.
A new study carried out at the Stanford Linear Accelerator Center (SLAC) explains why. The results could affect future quantum and photonics technologies in which light replaces electrons in computers and fluids in refrigerators, for example.
In QLED TV screens, dots absorb blue light and convert it into green or red light. At the low energies where TV screens operate, the conversion of light from one color to another is practically 100% efficient. But if the excitation energies are increased too much, which is necessary for brighter screens and other technologies, the efficiency drops off sharply. Researchers theorized as to why this happens, but no one had ever observed it at the atomic scale, nor could anyone adequately explain it.
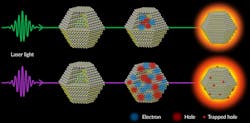
To see what was going on at the atomic level, SLAC scientists trained a high-speed “electron camera” on quantum dots as they were hit with energy laser pulses measuring in the millions of electron volts (MeVs). The researchers measured the dots’ behavior as they were hit with various wavelengths and intensities of laser light.
They found that the incoming laser light kicks electrons from the dot’s atoms and left “holes,” which were empty spots with positive charges that were free to move. The holes ended up becoming trapped at the dot’s surface and generating unwanted heat. To complicate matters, the electrons and holes recombine in a way that gives off even more heat. This increases the motion of the dot’s atoms, deforms its crystal structure and wastes even more energy that could have gone into making the dots brighter.
From the data gathered, the team tried to calculate and understand the interplay between electronic and atomic motions. The goal is to define the limits of photonic processes, such as light absorption and emission, at the limits of what thermodynamics allows. This could pave the way for technologies such as light-powered refrigeration, heating, cooling, energy storage and propulsion for spacecraft, as well as quantum computers.