An Engineering Refresher: The Laws of Thermodynamics
The Laws of Thermodynamics are the foundation of heat transfer and energy work. When any engineer is designing or implementing a system, the consideration of heat loss or energy produced is influenced by these fundamental principles. Reviewing these laws, while basic, provides us with an opportunity into how their general concepts affect our everyday designs.
The laws of thermodynamics were born during the Industrial Revolution of the 19th century in the United Kingdom and Europe. As new machine-driven manufacturing processes were being developed, physicists began studying the heat flow from machines that accompanied the work being produced. In an effort to gain maximum efficiency, attempts were made to create a perpetual motion machine; one that could run off its own heat it and operate indefinitely. However, what they discovered was that perpetual machines were impossible due to the inherent nature of work and heat loss, and from their analysis the laws of thermodynamics were founded.
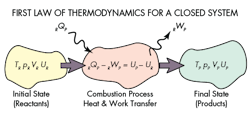
The First Law of Thermodynamics
E2 - E1 = Q - W
where:
E is the internal energy of a system
Q is the heat transfer into a system
W is the work done by a system
Julius Robert von Mayer, a German physicist, published papers in the 1840s that explained the modern day principles of conservation of energy and the first law of thermodynamics. The first law of thermodynamics states that energy can neither be created nor destroyed. Basically, it states that energy is conserved. According to NASA’s “Beginner’s Guide to Aeronautics," the definition of the first law is as follows:
“Any thermodynamic system in an equilibrium state possesses a state variable called the internal energy. Between any two equilibrium states, the change in internal energy (E) is equal to the difference of the heat transfer into the system and work done by the system.”
The internal energy (E) is a state variable, just like temperature or pressure, and is not dependent of any process. The first law of thermodynamics defines the internal energy is equal to the difference of the heat transfer into a system and the work done by the system. Internal energy is a form of energy like potential or kinetic energy. While conserving the total energy of the system, internal energy can be converted either to potential energy (stored in the system) or kinetic energy (for state changes).
Phase changes are obvious examples of the first law of thermodynamics such as when an ice cube melts in a glass of water. The ice melts to the next phase change as water and the overall temperature of the water lowers reaching an equilibrium state.
For a heat engine, the work output of the engine combined with the change in internal energy will equal to its heat input. Most heat engines operate in a cycle so the internal energy will have no net change.
Another example is refrigerator, which takes heat from a low temperature reservoir (the evaporator in the cooling compartment) and pumps it into the high temperature reservoir outside the refrigerator. The work input comes from the energy supplied via the wall outlet. The refrigerator output is the cooling air and the work it performs is equal to the energy supplied.
However, in real-world applications, some energy will always escape leading to inefficiency and the second law of thermodynamics.
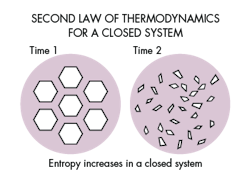
The Second Law of Thermodynamics
ΔS = ΔQ/T
where:
Q is the heat transfer into a system
S is the level of entropy
T is measure of temperature
A few years after the papers on first law of thermodynamics were published; German physicist Rudolph Julius Emanuel Clausius published an early version of the second law of thermodynamics in 1850.
The second law states that entropy of the universe tends to a maximum. Entropy is defined as the tendency for natural systems to break down and for energy in a system to be dissipated. Energy exhibits entropy in that it will continue to expand and move out. Entropy is the measure of disorder and randomness in a system.
The second law explains that while there are many possible examples of the first law, the only real world examples that occur are those in which entropy either remains constant or increases.
Entropy helps us realize that a system will seek out equilibrium. It helps describe, for example, that heat will flow from a high temperature to a low temperature system. In the example listed earlier with the ice cube, the temperature of the system (ice and water) reaches equilibrium as the ice reaches a phase change.
The second law of thermodynamics helps put the first law into context. Energy is never lost in a system, however, the energy available for work output will never be greater than the energy input. For example, an engine will not be able to transform all input energy into horsepower as some of the energy will be converted into heat and sound. The energy for the system as a whole is conserved but the usable energy is not.
Entropy is then a measure of the unusable energy in a closed system. As entropy increases the usable energy decreases by the same amount, hence the system is observing the first law and randomness increases.
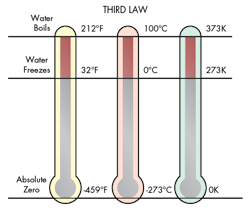
The Third Law of Thermodynamics
limT->0 S = 0where:
S is the level of entropy
T is measure of temperature
The German chemist Hermann Walter Nernst devised the third law of thermodynamics in 1905. The third law is a direct result of entropy. It states that at the temperature of absolute zero (0 degrees Kelvin), entropy will also approach zero.
All mater is in motion at the molecular level for the three major phases of matter. At absolute zero the motion of the average atom or molecule is zero. Since entropy is a constantly expanding, absolute zero is an unreachable extreme condition.
There have been instances of physicists coming close to absolute zero. At the Helsinki University of Technology Low Temperature Laboratory in 1993, a nuclear demagnetization device achieved a temperature of 2.8 x 10-10 degrees Kelvin. This translates to that a fragment equal to only 28 parts in 100 billion separated this temperature from absolute zero.
The third law reveals to engineers that refrigeration techniques must fail as you approach absolute zero. This is because refrigeration is a cycle process that requires the continuation expelling of heat. To maintain a constant low temperature, one connects a heatsink to the system to bring heat out of the system. Heat leaving the system indicates that entropy is decreasing. In a common home refrigerator the parameter is volume. When you compress the refrigerant in its gas phase it becomes a liquid in which heat is expelled. This refrigerant fluid is then deposited into the radiator.
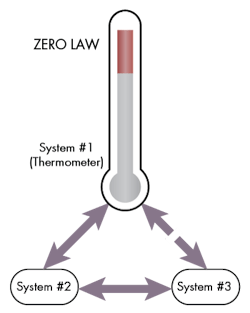
The Zeroth Law of Thermodynamics
The zeroth law of thermodynamics was developed after the first three laws had been established. British physicist Ralph H. Fowler invented the term for the law in 1935.
It is a basic concept but an important one as it states that if two thermodynamics systems are equal with a third thermodynamic system, then the first two thermodynamic systems are equal with each other as well. This law, while obvious, is necessary to define thermodynamic equilibrium and temperature.
Consider that system #1 and system #2 are in contact with each other and are in thermal equilibrium. Now system #2 is also in equilibrium with system #3. Initially there is no physical contact between system #1 and #3. But, if they are brought into contact, a thermal equilibrium is observed.
This is the principle that allows us to build thermometers. When a thermometer is placed in a system, the thermal property will change so that it can achieve thermal equilibrium and the indicated temperature on the thermometer will be equal to the initial system.
The equations listed here are simple general equations in how to determine entropy and internal energy. Each individual system will have its own heat transfer, internal energy, and entropy variables and equations. For a full analysis of a system, one is required to determine the values of temperature, pressure, volume, and time to develop full system equations.
Equations for the Laws of Thermodynamics
The First Law of Thermodynamics
E2 - E1 = Q - WThe Second Law of Thermodynamics
ΔS = ΔQ/T
The Third Law of Thermodynamics
limT->0 S = 0where:
E is the internal energy of a system
Q is the heat transfer into a system
W is the work done by a system
S is the level of entropy
T is measure of temperature
Looking for Parts? Go to Source ESB.