A major cause of failures in metal fasteners is electrochemical corrosion (rust), or so-called galvanic action. Engineers and designers alike can greatly reduce this type of corrosion by specifying fasteners made of the proper materials or with the right coatings.
All metals have an electrical potential. When metals with different potentials (steel and copper, for example) come into contact in the presence of an electrolyte such as water, it sets up a galvanic cell in which a low-energy current flows between the two metals. This galvanic action corrodes the metal with the higher potential. This makes it critical for engineers to know the potentials of the metals that are in contact—especially fasteners.
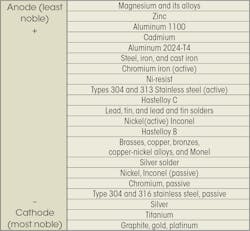
The accompanying chart shows the relative potential for galvanic corrosion for a number of commonly used fastener materials. The more widely separated two metals are on the chart, the most likely the metal with the higher potential will corrode. Usually, metals in the same group can be in contact without galvanic action taking place.
Here are some other tips for preventing electrochemical corrosion on fasteners and other components:
-Maintain constant stress in the faster. Irregular loading leads to corrosion.
-Separate dissimilar metals with a dielectric material (insulation, paint, or coatings).
-Avoid metal-on-metal situations in which the less-noble material has the smaller contact area.
-Current density and corrosion are greater when current flows from a smaller to a larger area. Because fasteners are always smaller than the rest of the assembly, they should be of the same material or lower in the galvanic series than the materials being joined.
You can use the galvanic process to your advantage by coupling parts to be protected to non-functional pieces of a less-noble metal. The less-noble parts (sacrificial anodes) will corrode instead of the protected part.