Strong, long-lasting adhesion depends more than just selecting the right adhesive. It also requires that the best surface preparation techniques be used. In this context, “surface” refers to the area and depth where the adhesive interacts with the substrate. The affinity of the adhesive with this surface ultimately determines bond strength. Whether bonding metals, plastics, or rubbers, proper surface preparation always plays an essential role in getting the best bond.
Bonds and failures
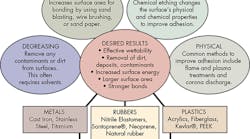
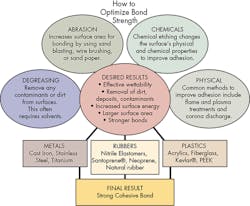
Adhesion can be mechanical, chemical, or a combination of both. Mechanical bonds, in large part, are determined by the area of the substrate in contact with the adhesive. Basically, roughening up the surfaces not only removes pre-existing deposits, but, more importantly, provides a larger surface area. The larger area translates into stronger bonds.
Alternatively, adhesives can attach to substrates chemically. In these bonds, the typical forces acting between substrate and adhesive include ionic, static, polar, and van der Waals forces. To get the best bonds, the surfaces must be free of all dirt, oils, and other contaminants.
Bonds can fail cohesively or adhesively. A cohesive failure, which is a breakdown of the adhesive itself, typically indicates the wrong adhesive that was used. Adhesive failures in which the bond between the adhesive and substrate fail are more difficult to assess. They could be due to inadequate surface preparation, improper adhesive, or substrates which inherently resists adhesive bonding. It is difficult, if not impossible, to get adhesives to bond to gold, for example. In a practical sense, if an adhesive is properly chosen and the substrate receptive to bonding, bond failure would be invariably due to insufficient surface preparation.
For good bonds, it is also important the adhesive effectively wets the substrate’s surface. Therefore, one goal of surface preparation is to increase the surface energy of the substrate to be bonded. Higher surface energies promote better wettability or the ability of a surface to absorb substances. Therefore, substrates with higher surface energies make for better adhesive bonds compared to lower surface energy substrates. This is another reason proper surface preparation is vital in getting easily wettable and cohesively strong surfaces with improved bond strength.
Degreasing
Degreasing is a principal step in surface preparation. It removes loosely held dirt and other contaminants from surfaces. Surfaces are often degreased with solvents such as acetone and methyl ethyl ketone. Depending on the substrate involved, alternative solvents such as isopropyl alcohol can also degrease surfaces. It is important to ensure all environmental, health, and safety regulations are met prior to selecting a solvent.
The most common methods of degreasing typically include two main steps:
1. The surface is either vapor degreased, wiped clean, or rinsed repeatedly, using the appropriate solvent, until the surface is free of all contaminants. How much this is done depends on the degree of contamination.
2. The surfaces are cleaned and dried completely to remove any residual solvent.
Abrasion
After degreasing, mechanical abrasion removes heavy loose surface deposits such as dirt, oxide layers, and other contaminants. Mechanical abrasion also increases the surface area for bonding, a critical factor. Some of the most effective mechanical abrasion methods include sandblasting, wire brushing, and abrasion with sandpaper or emery cloth. The surfaces must be cleaned both before and after abrasion to remove pre-existing contaminants.
A surface roughness RMS of 150 of 150 to 250 micro-inches is generally recommended for many metals. It is advisable to get parts machined to the proper roughness as early as possible in the manufacturing stage.
It is risky to rely solely on observation to determine whether surfaces are adequately roughened. It should be noted that abrasion techniques eventually employed might vary depending on the specific plastic, rubber, or metal being used. Depending on the material used, caution must always be exercised as some techniques may pose certain health hazards, such as roughening beryllium.
Chemical treatment
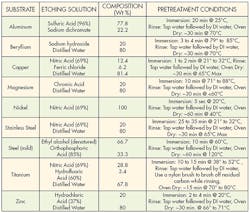
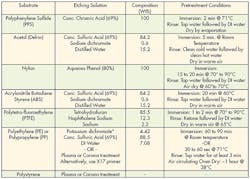
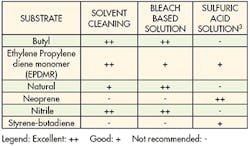
An alternative to mechanical treatment is chemical etching. Specific chemical techniques have been developed for treating different substrates. These treatments change the physical and chemical properties of the surface to improve adhesion. A wide array of various acids, bases and solvents are used for chemical etching.
Each substrate has a recipe that optimizes its bonding capabilities. These recipes and methodologies should be strictly followed to get the best adhesion while minimizing health hazards. Technicians should have good laboratory skills and take extreme care when handling chemicals. Wearing appropriate protective equipment and being thoroughly trained in handling chemicals is always recommended. And the appropriate environmental, health, and safety regulations must be followed when handling chemicals.
Physical methods
Often time, particularly with plastics, neither chemical treatment nor mechanical abrasion is effective. In these cases, techniques referred to as “physical methods” can change the plastic surface’s reactivity and modify it for better adhesion. Some common physical methods include:
Flame treatment involves exposing surfaces to be bonded to a gas flame for a few seconds. The flame oxidizes the surface and increases the surface energy by forming functional groups higher surface energies. Warping might be a potential downside to this method.
Corona discharges are usually generated by ionizing the air between two closely spaced electrodes. They react with substrate surfaces to form free radicals. These free radicals quickly react with oxygen
in the atmosphere and increase the substrate’s surface energy. This higher surface energy lets the surface be easily wet by the adhesive. Corona treatment is the most popular method of choice for preparing polyolefins and polyolefinic type materials for adhesives.
Plasma treatment differs from corona discharge and flame treatments in that it is typically carried out under partial vacuum. In plasma treatment, gas plasma activated by appropriate techniques creates a highly excited, ionized gas that reacts with the plastic substrate. Plasma treatments often tend to provide substrates with better stability than do corona discharge and chemical and flame treatments.
No matter which substrate and treatment is employed, the time period for adequate bonding is limited and depends on the technique. It is critical to determine the shelf life or window of opportunity for good bonding before the treatment loses its effectiveness. For example, plasma treating certain plastics might be effective for only a few hours. Hence, it might be a good processing procedure to treat parts on the manufacturing line.
Evaluating treated substrates
Once a surface is treated, it is important to know how effective the treatment was. The treated substrates can be evaluated in the following ways:
Destructive test: Mechanical strength tests such as tensile test or lap shear test can be performed to evaluate the mode and point of failure. Surface preparation is done to ensure failures occur in the bulk of the adhesive layer, not at the interface between adhesive and adherend. If the piece fails only cohesively, then good surface preparation techniques were employed. However, if it fails adhesively, the surface preparation technique carried out must be re-evaluated.
Non-destructive Tests: Water break test: Spray or coat a thin uniform film of deionized (DI) water on the treated substrate. A break in that film indicates there may be surface contaminants. This technique does not quantitatively analyze the surface.
Contact angle test: For quantitative analysis, measure the contact angle between the surface and a drop of reference liquid. The larger the contact anlge, the more wettable the surface.
UV light detection: Another way to detect surface contamination involves coating the surface with fluorescent oil and looking for contaminants under a UV light.
Surface preparation is critical for good adhesion. Fortuantgely, there are a fairly wide number of relatively simple and inexpensive techniques that can be used to prepare substrates. The proper surface preparation technique is usually dictated by the nature of the substrate. Other times, surface preparation could depend on safety issues. Ultimately, engineers must consider all approaches and choose the appropriate technique based on effectiveness, cost, safety, and the shelf-life of the substrate being treated.