With an aging population and an increase in natural disasters, it looks like the medical industry will need to scale for both day-to-day and for heavy fluctuations during disasters. Fortunately, industry is finding ways to use technology and even open-source community competitions to answer this seemingly inevitable future demand. Software, start-up competitions, and streamlining technology that already exists for the medical industry will provide a lot of value and reduce the time patients spend in medical care. This will reduce waiting times and cost, both of which will be imperative moving forward.
Going Paperless in the Medical Industry
The manufacturing industry is already working on streamlining documentation. Design history records, maintenance, and more can be digitized and even automated to ensure standards and safety concerns are met. Going paperless has a lot of benefit in streamlining production while maintaining documentation control. However, the medical industry is highly regulated. HIPAA and FDA requirements must be followed, among others.
Penumbra Inc., a global leader in interventional therapies, has selected Siemens Opcenter Execution Medical Device and Diagnostics (formerly known as Camstar Medical Device Suite) from Siemens Digital Industries Software to support its growing business and innovation goals. The solution, which enables paperless manufacturing in an FDA-regulated environment, will be the foundation for an operational platform designed to establish a more predictable manufacturing process and supply chain, while also providing the foundation for continued, rapid growth.
Automating and reducing paperwork not only save time, but can improve documentation. With manual documentation, verbiage could change from person to person and details could be forgotten between the time something happened and the time it is documented. If a person is filling out a document in a rush, notes might be short or miss important details.
“When we embarked on this journey, I was mainly interested in four areas: in-line data verification, elimination of paper storage, reduction of resources doing DHR [Device Health Record] review, and the ability to collect and respond to real time data,” said Ryan Powers, VP of operations at Penumbra. “The implementation resulted in process improvements and efficiencies that were not anticipated, and the user interface has had a significant impact on quality of life for the product builders.
“With Siemens’ MES, we will have even better enforcement and visibility into our existing processes, which will in turn help to improve efficiencies, reduce costs, and improve the reliability and quality of manufacturing,” he continued.
Some features to look for in digital platforms beyond meeting regulations for its applications are integration, flexibility, and support. The massive amount of data that a platform needs to have loaded into it, and the ability to work “out of the box”, can be challenging. Like a smartphone app, software should be almost self-explanatory and require minimal or no training. However, a software provider should also have after sales or training resources in case there are features a user might not inherently find that may increase the software’s value.
While preset features can help users get started faster and ease integration, software should also be flexible. Having the ability to change or customize the software to specific needs or applications will let features evolve with needs over time.
"We’re impressed with the modern platform and out-of-the-box capabilities for medical devices from Siemens Opcenter that can easily be configured to mold to our manufacturing operations,” said Pankaj Tiwari, VP of information technology at Penumbra. “The Siemens team did a great job in guiding and helping us to best leverage the system to meet our needs.”
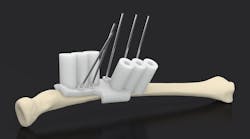
Drill guides can be customized for patients. This show doctors where to drill or cut. This can make an operation go faster and smoother without the doctor having to free-hand holes or incisions.
Streamlining Production In the Medical Industry
Materialise was founded in 1990 and has become a driver of 3D printing technology in the medical industry. With the same challenges previously mentioned, the Materialise Mimics Care suite was designed around the existing workflows of hospital clinicians or surgeons across multiple disciplines. It’s an open and flexible platform you can use with any existing imaging or printing system.
This platform helps streamline the imaging data to produce 3D anatomical models or certified implants and devices. Every body is different, so having models and implants that match exactly to each patient means doctors can better prepare for surgery, reducing time in the operating room. Traditional manufacturing processes can take a lot of time and cost to make implants, and they might not precisely match the geometry or contours of the area it is mating with, or the natural part that it is replacing. 3D-printed implants can match better, reducing time in the operating room and in recovery.
There are many benefits to 3D printing in the medical industry. With the increase of 3D printed manufacturing, keeping everything HIPAA- and FDA-compliant while keeping up with demand will be imperative as demand scales. Medical software will be needed to keep medical facilities organized. For example, if a bad batch of material is discovered, platforms such as Materialise can track down every part made with that batch to notify clients.
Medical software sounds complex, and it can be, but it can be as simple as making recovery from a broken bone less annoying. TriMed announced the availability of Exiom’s innovative Xkelet casts and product line for the U.S. medical marketplace on October 8th. The orthosis system is recognized for its custom-fitting 3D printed cast that optimizes hygiene, functionality, and comfort. The Xkelet brace fits perfectly on children or adults and comes in virtually any color.
Plaster casts can make simple tasks like showering difficult. New open 3D-printed casts can reduce atrophy and the annoyance of traditional casts. (Credit: TriMed)
It starts with imaging, but an MRI or x-ray isn’t needed. A scanner is passed around the patient’s broken limb to create a 3D model. The software calculates the cast’s length, width, height, and volume. The cast is then 3D printed with bio-compatible materials. Additionally, the system controls stiffness and density of the part. TriMed says these features and design eliminates the loss of muscle tone and recovery associated with plaster casts. The cast’s open lattice design is lightweight, waterproof, and breathable so the patient’s skin receives proper air circulation.
“Anyone who has suffered a fractured or broken arm or wrist can attest to the sheer discomfort associated with a rigid plaster or fiberglass cast,” said Becky Dobrinich, TriMed CEO and co-founder. “TriMed takes the orthopedic cast industry into the 21st Century with scanning technology, 3D printing, and the highest level of customization for the patient.”
The more customized care the industry is capable of means there could be an expected increase in medical documentation and need to automate and streamline information with software solutions such as the ones these companies have developed.