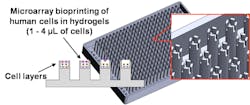
With the first demonstration of microextrusion-based printing of living cells in 2003, three-dimensional (3D) bioprinting has taken front stage in the development of manufactured human tissues. This technology specifically targets the creation of human tissue replicas using “bioinks,” which are mixtures of human cells, hydrogels, growth factors, and extracellular matrices (ECMs). The bioinks can then be “printed” for precise placement of human cells using a bioprinter, which can have different printing mechanisms, such as piezoelectric, microsolenoid valve-based, microextrusion-based, and laser-assisted printing.
Traditional two-dimensional (2D) cell cultures lack the ability to mimic cellular microstructures and microenvironments required for cell functioning that resemble normal tissues. Current 3D bioprinting approaches have demonstrated that 3D-printed tissues can be used as disease models for predictive drug screening. There has been an explosion in interest for 3D-printed human tissues with its global market size estimated to be $295 million in 2016, which is expected to grow to $1.8 billion by 2021. These values are based on the applications of 3D-printed tissues, which include disease tissue modeling, toxicology testing, tissue engineering, and skin transplants.
For example, tumor cells extracted from biopsy samples can be printed and matured in the laboratory to create patient-specific tumor replicas, which can then be used to screen combinations of therapeutic drugs to create individualized cancer treatments. Although there are still several technical challenges, there is a bright future for 3D bioprinting technology.
Need for Bioprinted Tissues in Drug Discovery
As the discovery of new compounds that could have harmful or therapeutic properties continue to rise, research models are needed to properly study the effects of these compounds. Animal models represent the gold standard for biological testing; however, these models are low-throughput and face increasingly larger ethical concerns. The development of manufactured tissues allows scientist to test a large number of samples without the drawbacks associated with animal testing. To utilize manufactured tissues for research purposes, the constructed tissue must behave biologically normal prior to being tested with compounds or other conditions.
The traditional methods of testing compounds using human cell samples in preclinical evaluations have been limited to using two-dimensional (2D) cell cultures. Since the cells in these cultures lay completely flat on a surface as a monolayer, they lack the tissue microstructures and microenvironments required for cell functioning. Research in artificial tissue constructs has shown that the microenvironment in which the cells grow in is just as important as the cells themselves. This has led to the development of three-dimensional (3D) cell cultures, which aim to provide the correct environment that allow the cells within them to mimic their behavior in vivo. However, current methods of 3D cell culture face a multitude of challenges as well. These methods utilize…
- Low adhesion plates
- Hanging drop plates
- Microfluidic chips
In particular, the microfluidic chips have microchannels and chambers, which funnel their cells on top of each other to allow them to interact in three dimensions. The premise of these 3D cell cultures is based on utilizing the geometry of the medium or vessel that hold the cells. This allows cells to interact with each other in three dimensions, providing a more in vivo-like environment. However, a major drawback to these methods is that they are relatively low-throughput and still have difficulty truly mimicking in vivo conditions.
3D bioprinting aims to fill the gaps found in 2D and 3D cell cultures. This process places cells in a more natural 3D environment that previous 3D cell culture systems have difficulty doing. This is done by dispensing bio-inks as a liquid but can be manipulated by some outside force (e.g. ultraviolet light, change in temperature, etc.) that turns it into a semi-solid gel due to the hydrogel’s material property. Then a new layer can be printed on top of the newly formed gel layer, leading to a 3D tissue produced by a layer-by-layer method. These cell layers are then allowed to grow, to differentiate and to induce cell-to-cell interactions to form tissue-like structures or be subjected to compounds for testing and processed for analysis or imaging.
The Process of 3D Bioprinting
A. Bioinks
Although the process of 3D bioprinting is fairly simple, various numbers of available bioinks, bioprinters, cell culture/maturation and staining protocols, and cell imagers make it difficult to choose which specific component is best suited for a specific need. By examining each specific component, this provides greater insight on how 3D bioprinting can be best utilized. Beginning with bioinks, its liquid property prior to any curing is the major advantage that places 3D bioprinting above other methods. By being a solution, this allows a scientist to control the number of cells and other additives including growth factors and extracellular matrices (ECMs) to be printed. The hydrogels in these bioinks are then cured by exploiting their ability to assemble into structures that suspend cells and spheroids in 3D, called polymerization. Polymerization can vary between different hydrogels, with the most common being photopolymerization, ionic polymerization, and temperature-responsive polymerization. Depending on which process is selected, curing additives (a.k.a., crosslinkers) are needed, but could be potentially toxic to the cells within the bioinks. This can be resolved by using gentler polymerization processes, but this generally leads to longer polymerization times or weaker gel structures. Therefore, sacrificial and support bioinks can be used to provide temporary or permanent support until the hydrogel can fully cure.
B. Selection of Human Cells
The choice of human cells in 3D bioprinting is crucial for cell proliferation and functioning, ultimately for recapitulating human tissues in vivo. For example, tumor tissues comprise multiple malignant and surrounding cell types with different biological functions. In addition to the cancerous cell types, most tumor tissues contain normal surrounding cell types that provide supportive, structural or barrier functions, are involved in vascularization, or provide a niche for cancer stem cell maintenance and differentiation. Understanding the tumor-specific cell composition and localization of ECM components in the tumor tissue of interest is required to better engineer tumor tissues with specific physiological functions. These ECM proteins provide strength and space-filling functions, bind growth factors, regulate protein complexes, and promote cell adhesion and proliferation in cellular signaling. In general, tissue structures are generated by printing human cells and ECM components of a tissue found in vivo (a.k.a., biomimicry) or dispensing stem cells to replicate the desired biological micro-tissue architecture and functions by differentiating the stem cells into multiple cell lineages, thus reproducing ECM components using appropriate cell signaling (a.k.a., autonomous self-assembly).
C. Methods of Cell Printing
After preparing the bioink, the solution can then be “printed” for precise placement of human cells using a bioprinter, which can have different printing mechanisms, such as piezoelectric, microsolenoid valve-based, microextrusion-based, and laser-assisted printing (Fig. 1). These methods represent three major technologies used for 3D printing: inkjet/droplet, extrusion, and laser. The inkjet/droplet approach focuses on using a technique that creates variations in pressure to form droplets of bioinks. These droplets are then precisely placed layer-by-layer on a substrate to create the tissue. However, it is limited to using bioinks that are not highly viscous. Extrusion technology uses some type of pneumatic or mechanical dispenser to release a stream of bioink onto a substrate. This approach is not limited to viscous bioinks, but the cells in these bioinks do suffer larger amounts of sheer stress. Lastly, the laser approach pulses a laser beam at a glass slide coated in a laser-absorbing layer and a layer of cells and biological materials. The laser excites these layers, discharging droplets containing cells. This approach faces neither limitation from the two other methods, but large tissues are difficult to create due to the width of the laser and metallic residues may contaminate the sample.
1. Photographs of a microsolenoid-based bioprinter and a dispensing head with six microsolenoid valves for dispensing biological samples.
D. Methods of Cell Analysis
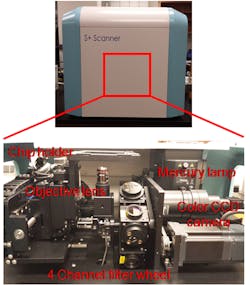
Lastly, after creating human tissue replicas, they could be exposed to test compounds, stained with fluorescent dyes, and analyzed to measure a myriad of cellular processes, including cell viability/cytotoxicity, cell differentiation, ECM expression, changes in nuclear function, apoptosis/necrosis, mitochondrial membrane potential, oxidative stress, intracellular calcium levels, glutathione levels, etc. Cell analysis is often done using fluorescent microscopes on cryosectioned tissues (Fig. 2). This method flash freezes bioprinted tissues to preserve their natural structure, which is then sectioned into thin layers and dyed to visualize the structure within the tissues. Cryosectioning is required as current imaging processes are poorly suitable to peer inside the bioprinted tissues because they are too thick for imaging and covered with surrounding cells. A major drawback to these methods is due to its inherent nature of low throughput. Thus, in situ tissue imaging is urgently necessary to better understand biological changes during cell maturation and after compound exposure.
2. Photographs of an automated fluorescent microscope with four filter channels and a CCD-based detector for high-throughput image acquisition.
Quality Control of Bioprinted Tissues
Prior to using the data obtained from bioprinted tissues, it is important to check if this data is reliable and correlates well with tissues existing in the body. It is the main goal of any cell culture to be able to recapitulate tissue structure in vivo and to mature into functional tissue. For example, the liver is a major detoxifier in the body because it produces numerous drug-metabolizing enzymes that break down drugs and other harmful compounds. Drugs such as acetaminophen (an active ingredient of Tylenol) often have found to have adverse side effects because they are transformed into toxic metabolites by the enzymes in the liver. Traditional 2D cell cultures of liver cells have difficulty in detecting these reactive species because the enzymes have low expression or liver cells derived from individual donors suffer from high lot-to-lot variation. Bioprinted liver tissues and other 3D-cultured liver cells have been able to maintain high levels of drug-metabolizing enzymes and behave more in vivo-like, thus better predicting adverse drug reactions in the livers.
Clinical Applications of Bioprinted Tissues
In addition to its applications to generic drug screening, 3D bioprinting has a bright future in filling a clinical need by being able to create tissue that will exactly mimic a patient’s biology, allowing for drug treatment courses that are specifically catered for them. When new drug candidates are tested in their preclinical evaluation phases, the cells tested in these studies are standardized throughout the entire pharmaceutical industry. However, a patient’s cells may not behave the same when being subjected to these drugs, particularly if the patient requires a cocktail of drugs to treat an illness. Here, 3D bioprinting technology can be used to create tissue samples from a small number of cells that are harvested from the patient. For example, tumor cells extracted from biopsy samples can be printed and matured in the laboratory to create patient-specific tumor replicas, which can then be used to screen combinations of therapeutic drugs to create individualized cancer treatments. With an extremely low success rate of gene-targeted cancer therapy, the 3D bioprinting approach could be a good alternative.
Estimated Market Size of Bioprinted Tissues
As the technology begins to develop, the interest in 3D bioprinting only continues to grow. This is visible as its global market size is estimated to be $1.8 billion by 2021 from $295 million in 2016, according to BCC research. This explosion in market value is due to the clinical applications that will become available by 2021. In addition to oncology, the cardiovascular, orthopedic, and plastic surgery field will greatly benefit from 3D bioprinting as its ability to print tissue grafts provide greater medical advantages compared to traditional methods. Although the applied and research markets will not reach the same valuation as the clinical industry, it is still expected to more than double in the next five years.
Current Technical Issues of 3D Bioprinting
With any new technology, there are technical pitfalls that must be overcome. One of the major issues facing the 3D bioprinting field is the lack of angiogenesis within these tissues. The human body is able to regrow and maintain its organs with the vast system of blood vessels that help bring nutrients and carry away wastes. However, current 3D bioprinting methods are unable to print blood vessels in these constructs; therefore, the tissues must use diffusion as the main source of exchange. This roadblock limits bioprinted tissues to relatively small volumes (< 1 cm3). It is possible to create larger tissues with contemporary methods, but the cells in the center of the tissues printed will suffer from a lack of nutrients, creating a region of cell death called “zonation”. This may be a blessing in disguise, as the research market will be looking for a system that will allow for rapid testing and combinatorial optimization of microenvironments. Many of the materials used in the bioinks are extremely expensive, which push researchers to develop miniaturized systems that minimize wastes and allow for large numbers of samples to be tested. In addition, many of the current platforms are poorly suitable for long-term cell culture, which becomes a compounded factor to cell death when measuring the toxicity of new compounds.
Miniaturized 3D Bioprinting
This is only made worse as these platforms are also poorly suitable to provide high-throughput screening. These challenges provide an opportunity for new technology to be developed. The below images represent a cutting-edge 384-pillar plate for miniaturized 3D bioprinting that can be placed within a standard 384-well plate (Fig. 3).
3. (Top image) Schematics of a 384-pillar plate with sidewalls and slits (384PillarPlate) for bioprinted tissue cultures. (Bottom image) Photographs of an injection-molded 384PillarPlate loaded on a bioprinter for layer-by-layer cell printing and culture.
The gaps located at the top of the pillar provide an area in which the bioink can be printed. The walls surrounding the gaps help hold the bioink in liquid form prior to polymerization and have spacing between them to allow for diffusion of nutrients when the manufactured tissues are submerged in a media solution required for cell growth or differentiation. This system can also be used without an expensive 3D bioprinter as it has been shown to be able to create tissues by simply dipping these pillars into a bioink and polymerizing the remaining bioink in the gaps of the pillar tops. The 3D cell cultures created in this platform is also able to be screened in high-throughput because of its ability to be paired with standard 384-well plates, addressing many of the issues with current 3D cell culture platforms. Although the technology is not fully perfected, it shows great promise to create miniaturized human tissues and advance the field as a whole.
Conclusively, 3D bioprinting is still in a relatively fledging state, but its impact on research and clinical applications are paramount. It has already shown to be more accurate and representative of in vivo tissue cultures and aims to be tenable to current high-through screening methods. Challenges in this field only serve to be opportunities to expand. We are still far from printing a beating heart, but it is not outside the realms of possibilities.